2025-06-11
Cell Regeneration | Breaking Traditional Barriers: Deep Learning Unlocks a New Dimension in Cellular Mechanics
Cell Regen | 突破传统瓶颈!深度学习解锁细胞力学新维度
Cell mechanical properties are critical biophysical markers that reflect cell phenotype and function. They play an essential role in elucidating cellular behaviors, understanding disease mechanisms, and advancing the development of cell-based therapies. However, current single-cell mechanical assessment methods based on atomic force microscopy (AFM) face significant limitations. On one hand, their throughput is extremely low, with only a few dozen cells analyzed per hour. On the other hand, the technique requires complex instrumentation and expert operation, making it unsuitable for high-throughput screening of large-scale cell samples. These constraints have become a major bottleneck in both basic research and clinical translation.
To address this challenge, Professor Yanan Du's research group from the School of Biomedical Engineering at Tsinghua University recently published a study titled "Image-based evaluation of single-cell mechanics using deep learning" in the journal Cell Regeneration. The team developed an innovative deep learning-based method for evaluating single-cell Stiffness using bright-field images. This approach enables efficient and accurate assessment of cellular Stiffness at the single-cell level. It offers a promising tool for research and clinical applications in cell mechanics, with the potential to overcome the limitations of traditional techniques and drive progress in the field.
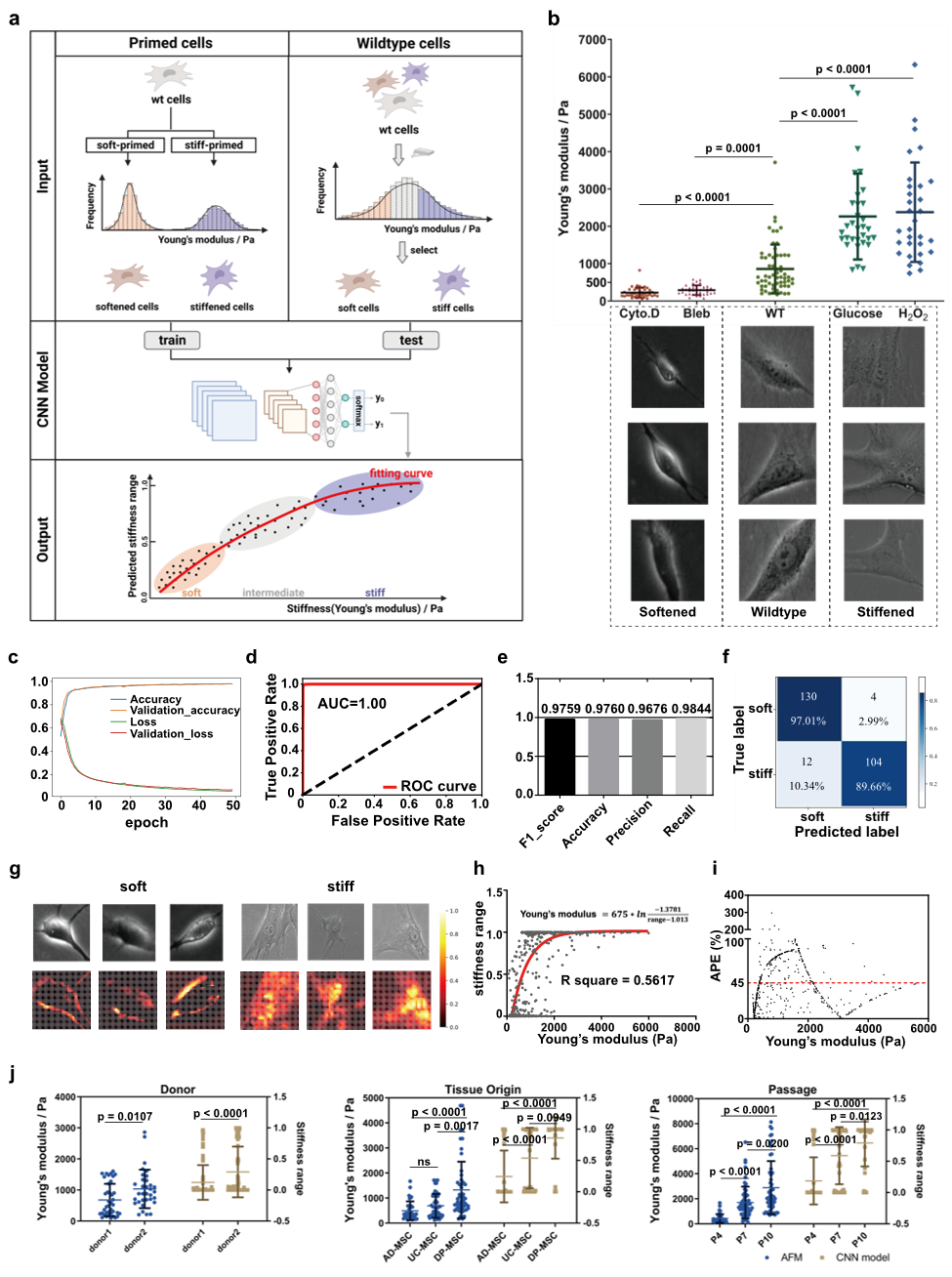
Figure 1 Stiffness Classification Model and Its Performance
This study introduces an innovative deep learning-based approach for evaluating cell Stiffness. By constructing a convolutional neural network (CNN) classification model, the researchers achieved efficient and accurate classification of the Stiffness of mesenchymal stem cells (MSCs) and RAW264.7 macrophages, and further investigated the correlation between cell Stiffness and cellular function. MSCs were treated with various compounds to generate a subpopulation image dataset spanning a Stiffness range of 200 Pa to 3 kPa, resulting in over 60,000 bright-field images of softer and stiffer MSCs, respectively. The CNN-based Stiffness classification model demonstrated excellent performance on the test set, achieving an AUC of 1.00, an F1 score of 0.9759, and an accuracy of 0.9760. In addition to accurately predicting the Stiffness range of wild-type MSCs, the model showed a comparable mean absolute percentage error (MAPE) to that of atomic force microscopy (AFM), indicating strong reliability.
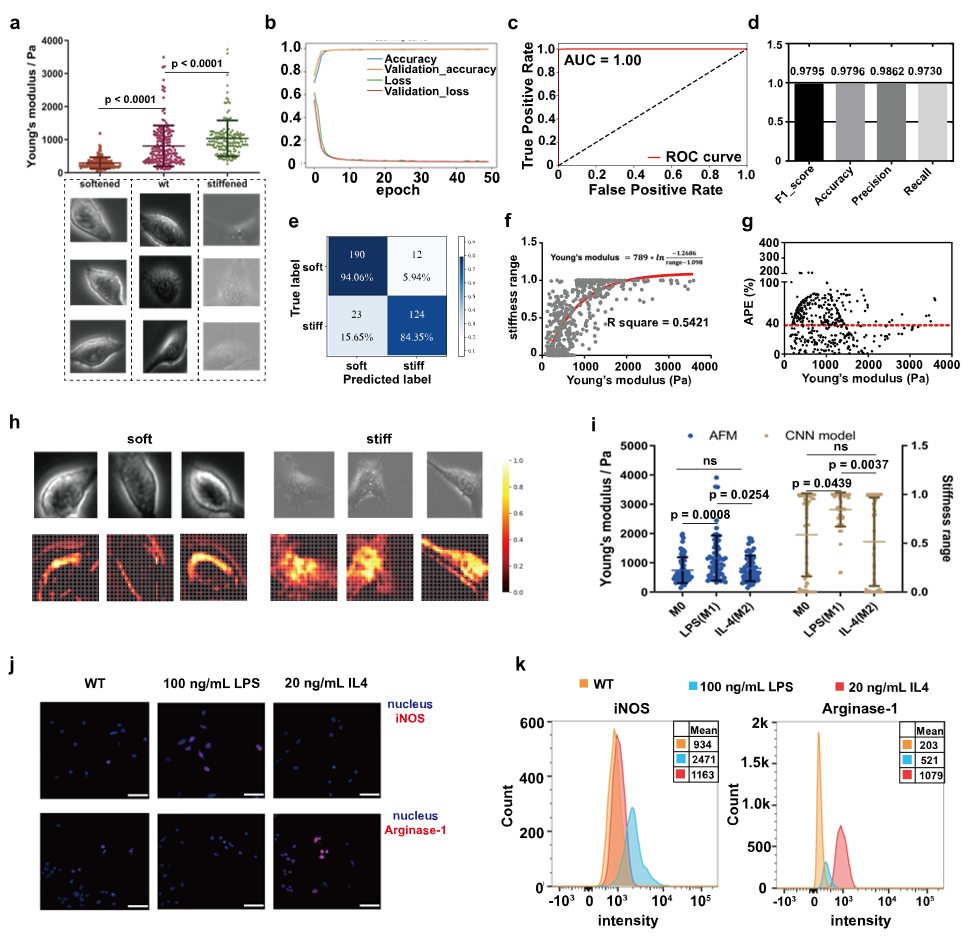
Figure 2 Stiffness Classification Model for RAW264.7 Cells
To further validate the generalizability of the Stiffness classification model, the researchers conducted experiments using the RAW264.7 macrophage cell line. Soft and stiff cell subpopulations with Stiffness values of approximately 200 ± 100 Pa and 2 ± 1 kPa, respectively, were generated through specific treatments. Over 60,000 single-cell images were collected for each group to construct the dataset. The trained CNN-based Stiffness classification model again demonstrated strong performance on the test set, achieving an AUC of 1.00. The model's predictions also showed good agreement with atomic force microscopy (AFM) measurements, with a comparable mean absolute percentage error. These results suggest that the deep learning-based, cell type-specific Stiffness classification model is applicable for evaluating Stiffness across different cell types.
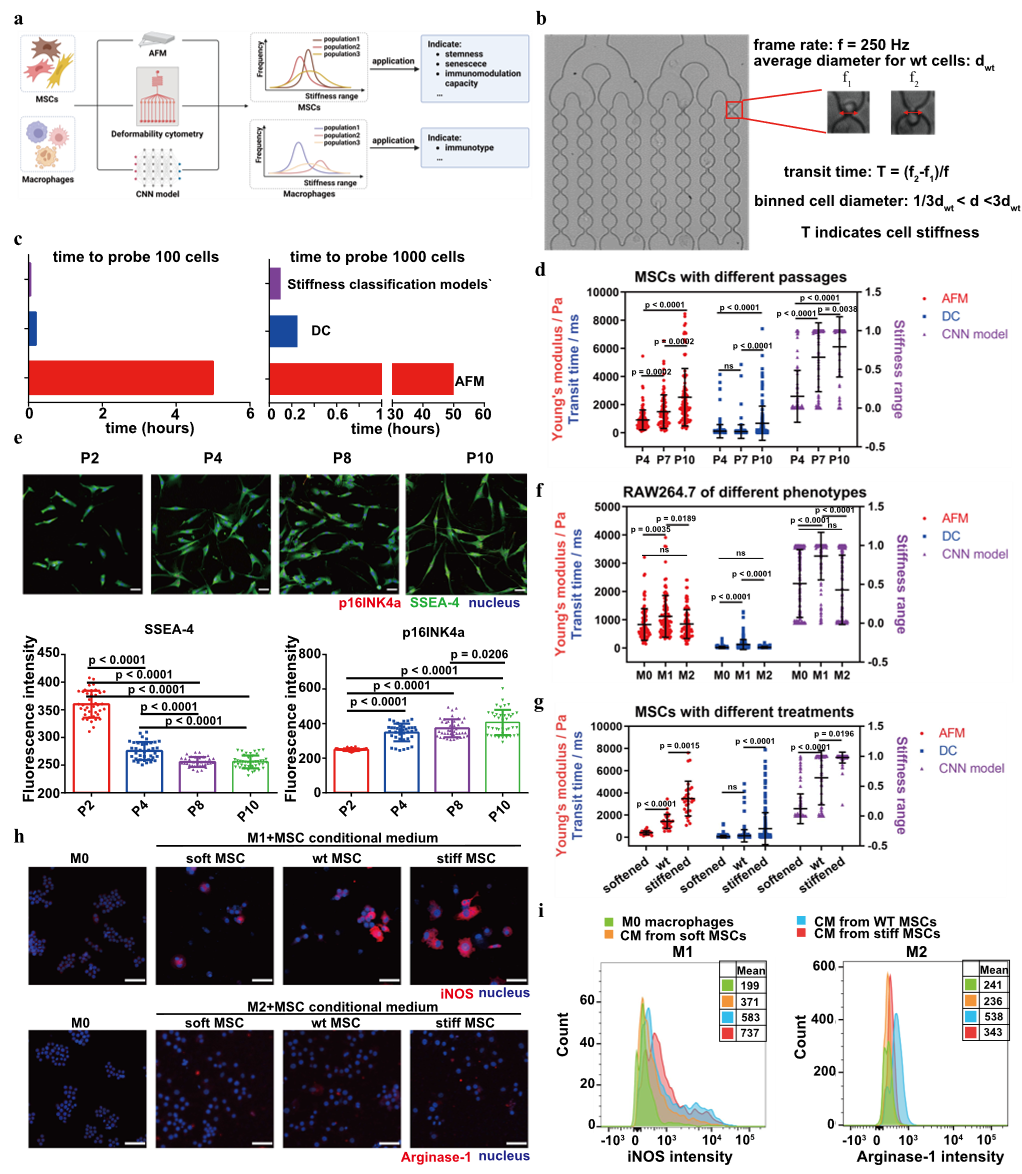
Figure 3 Comparison of Stiffness Classification Models and Functional Analysis
The researchers compared the CNN-based Stiffness classification model with deformability cytometry (DC) and atomic force microscopy (AFM) in terms of efficiency and accuracy. In studies involving MSCs at different passages, all three methods consistently indicated an increase in cell Stiffness with higher passage numbers. However, DC failed to accurately detect Stiffness differences among all MSC passages. Further functional analysis revealed that MSC Stiffness is closely associated with stemness, senescence levels, and immunomodulatory capacity. In macrophage studies, all three methods consistently showed that M1 phenotype macrophages exhibited higher Stiffness compared to M0 and M2 phenotypes. These findings suggest that the CNN-based Stiffness model holds strong potential for investigating cell function.
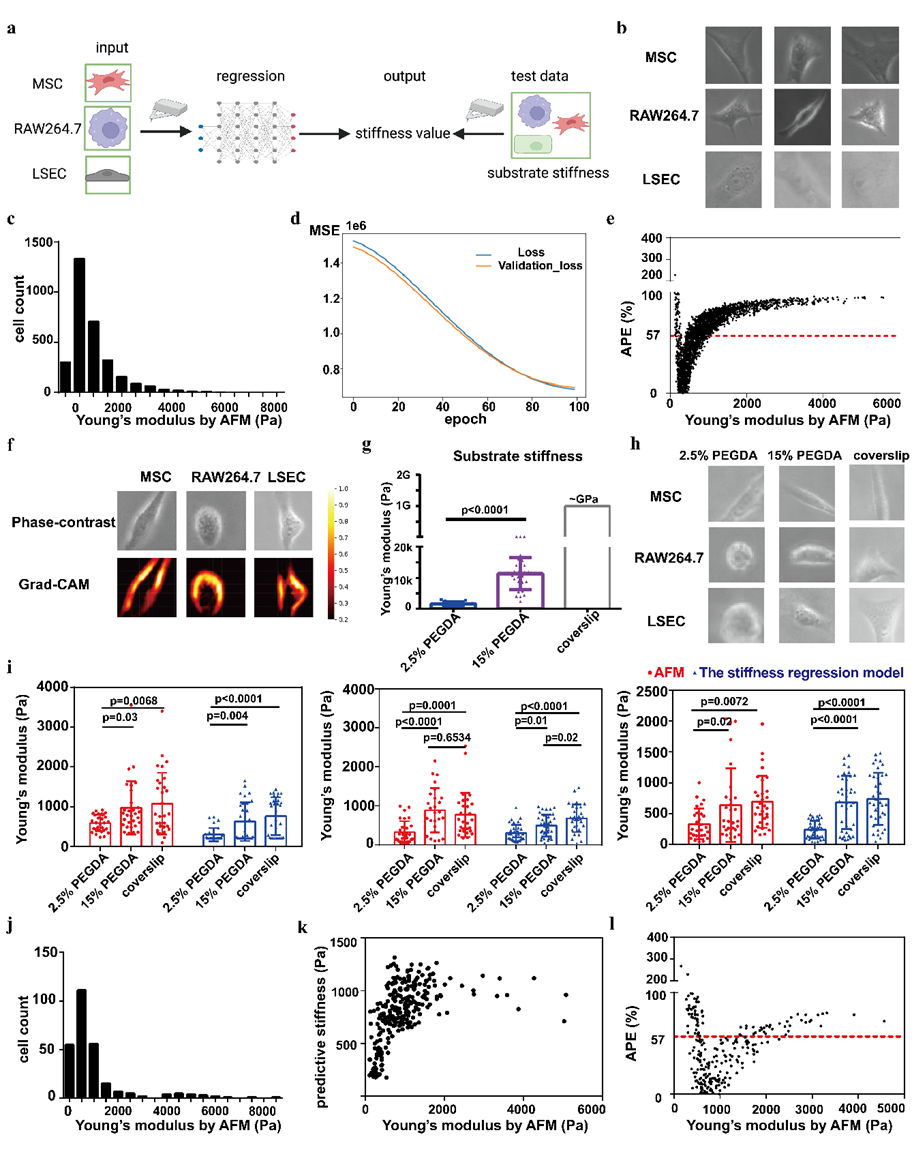
Figure 4 Stiffness Regression Model and Performance
Building upon the Stiffness classification model, the researchers developed a more refined deep learning-based regression model to directly predict Stiffness across different cell types. The dataset included images of 1,149 MSCs, 1,237 RAW264.7 cells, and 939 liver sinusoidal endothelial cells (LSECs), with single-cell Stiffness values obtained through AFM measurements. The model was trained using mean squared error (MSE) as the loss function, achieving an average percentage error (APE) of 57%. The model successfully predicted Stiffness differences in cells cultured on substrates of varying rigidity, indicating its potential for cross-cell-type Stiffness assessment. This serves as a proof of concept for future efforts to expand the dataset and optimize the model architecture to enhance prediction accuracy.
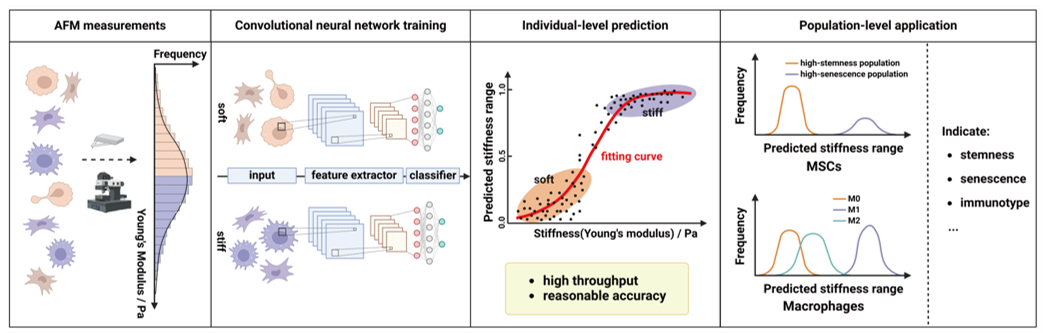
Figure 5 Deep Learning-Based Stiffness Modeling Strategy
In summary, this study established a CNN classification model that enables efficient and accurate classification of Stiffness in MSCs and RAW264.7 cells, while also exploring the relationship between cell Stiffness and function. The subsequent proof-of-concept regression analysis demonstrated that, despite a limited dataset, it is feasible to predict Stiffness differences across cells. Looking ahead, with expanded datasets, optimized models, and exploration of more cell types and mechanical properties, deep learning-based image evaluation models hold great promise in the biomedical field, particularly in advancing the standardization and functional assessment of cell therapy products.
Professor Yanan Du from the School of Biomedical Engineering at Tsinghua University is the corresponding author of this article. Zhaozhao Wu, a PhD student (Class of 2017) from the School of Biomedical Engineering, and Yiting Feng, a PhD student (Class of 2022) from the School of Basic Medical Sciences, are co-first authors. Professors Huijun Chen from the School of Biomedical Engineering and Yan Shi from the School of Basic Medical Sciences also made significant contributions to this work. This research was supported by multiple grants from the National Natural Science Foundation of China.
Correponding Author
Yanan Du is a PhD advisor and currently a Professor at the School of Biomedical Engineering, Tsinghua University. Her research focuses on the interdisciplinary field of microtissue engineering, where she conducts innovative work bridging theoretical exploration and technological translation. The 3D microtissue technologies developed by her group serve as next-generation platforms for the amplification and preparation of cell-based therapeutics, as well as advanced drug delivery systems, revolutionizing in vitro cell culture and regenerative medicine. These technologies also support the construction of biomimetic physiological and pathological models for high-throughput drug screening and disease mechanism studies. Professor Du has published numerous high-impact research articles, and several microtissue engineering products from her lab have been commercialized, having obtained certifications from both the U.S. FDA and the China National Medical Products Administration. Her work provides novel platform technologies, theoretical models, and solutions for regenerative medicine, drug development, and pathology research.

DOI: https://doi.org/10.1016/j.engmed.2024.100038

