2023-11-15
Advanced glycation end products mediate the extensive crosslinking of the extracellular matrix in cirrhotic liver tissue|NBME News
Orit Kollet & Irit Sagi
Department of Immunology and Regenerative Biology, Weizmann
Institute of Science, Rehovot, Israel.
Liver cirrhosis--a severe and irreversible condition associated with liver dysfunction, and a leading cause of morbidity and mortality worldwide1--is characterized by dysregulated remodelling of the extracellular matrix (ECM). Cirrhosis develops as a progression in the fibrotic scarring of liver tissue, caused by chronic pathological hepatic damage owing to the excessive accumulation and disorganization of collagen and other matrix fibrillar proteins2. The main risk factors for liver fibrosis and cirrhosis are infections leading to chronic hepatitis B and C, excessive alcohol consumption, type 2 diabetes, non-alcoholic fatty liver disease, and several other autoimmune and metabolic disorders. It is understood that the ECM-remodelling enzyme lysyl oxidase (LOX) mediates the intensive and imbalanced crosslinking of collagen fibres in fibrotic tissues3 . However, the failure of current LOX-targeting therapy in clinical trials has prompted the exploration of alternative enzymatic and non-enzymatic protein-crosslinking mechanisms such as transglutaminase and advanced glycation end products (AGEs) respectively, that may contribute to the abnormal build-up of ECM and fibrosis. AGEs are produced by non-enzymatic glycation when reducing sugars react with proteins or lipids--a biochemical process that progresses with age. AGEs form and accumulate both inside and outside of cells, pathologically altering protein structure and function while remaining highly stable. They disrupt ECM metabolism, induce pro-oxidant and inflammatory processes, and are implicated in the pathogenesis of cardiovascular diseases, Alzheimer's disease, renal failure, diabetes mellitus and other age-related chronic diseases4 .
Collagen crosslinking by glucose alters the molecular organization and charge distribution in type I collagen fibrils5. In fact, the roles of such collagen glycation in tissue, and of AGEs and their receptors, in the progression of non-alcoholic fatty liver disease have been well established6. However, the relationship between the degree of collagen glycation in disease conditions and the underlying mechanisms of pathogenesis is not fully understood. In particular, the specific contributions of AGEs to collagen-crosslinking-derived liver cirrhosis are unknown. Furthermore, the complex chemical reactions required for the formation of AGE-crosslinked ECM, and the diversity of crosslinking structures that can exist concurrently, hamper the study of AGE-crosslinking mechanisms in vitro. It is thus critical to distinguish and segregate the contributions to molecular crosslinking of the three main crosslinking mediators: LOX, AGEs and transglutaminase. Now reporting in Nature Biomedical Engineering, Yanan Du and colleagues describe a method that combines liver-tissue decellularization and liquid chromatography–tandem mass spectrometry (LC–MS/MS) to quantify the degree of crosslinking in the ECM without interference from non-ECM components7. The method allowed the authors to find that AGEs play a central role in mediating collagen crosslinking in the ECM of cirrhotic liver tissue (Fig.1).
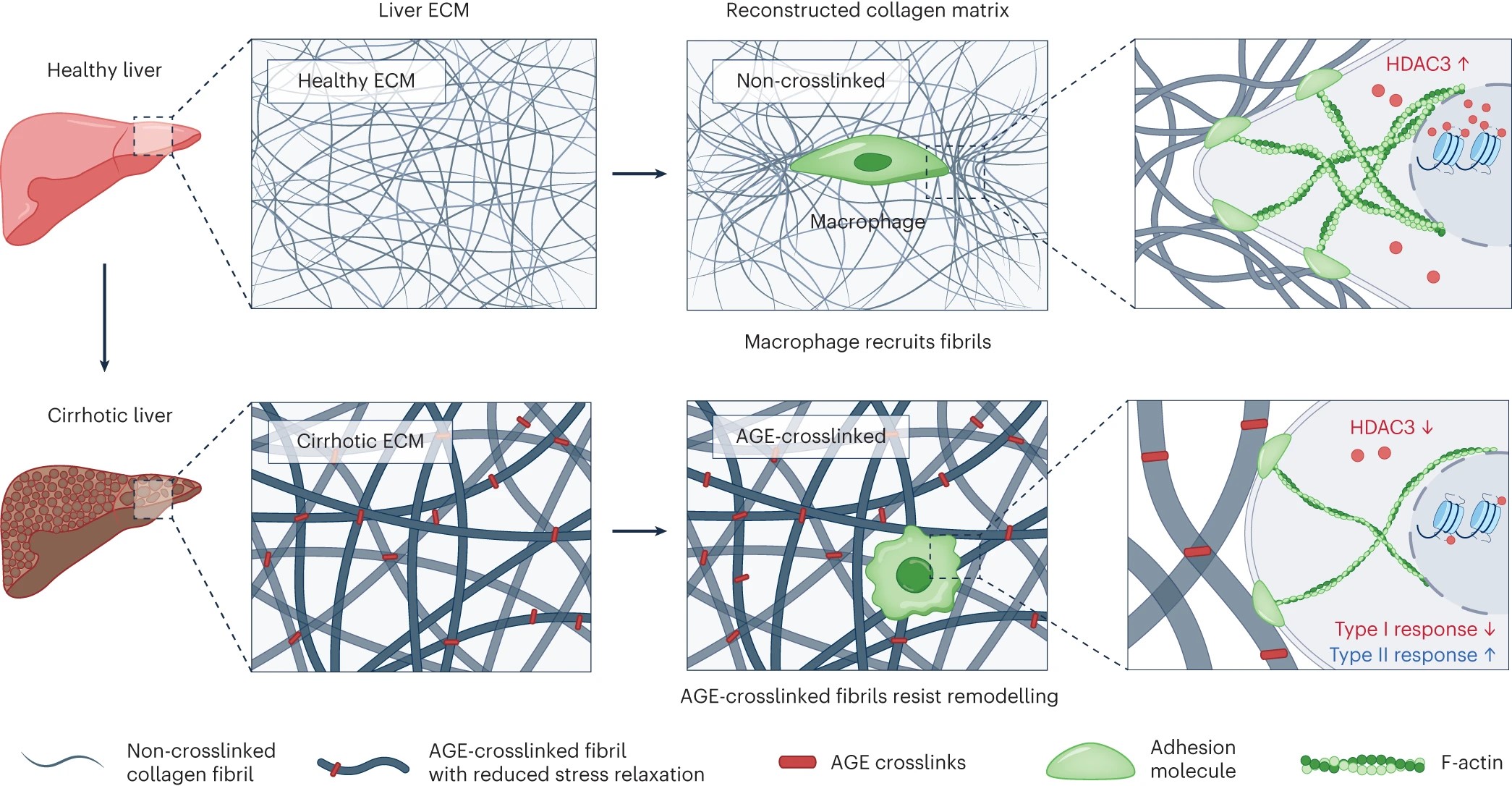
Fig. 1|Matrix crosslinking mediated by AGEs. Left: the ECM in cirrhotic liver has increased elastic modulus, owing to tighter entanglements between collagen fibrils. Middle: in vitro reconstruction of an AGE-crosslinked collagen matrix recapitulated the main characteristics of fibrotic liver ECM in vivo7. Right: AGE-crosslinked fibrils show lower stress-relaxation rates, and therefore resist macrophage-mediated remodelling. Fewer AGE-crosslinked fibrils being recruited by macrophages causes downregulation of the expression of adhesion molecules, decreased formation of protrusion structures, lower cytoskeleton organization and lower expression of histone deacetylase 3 (HDAC3) in the macrophages, as well as downregulated type I immune responses and upregulated type II immune responses. F-actin, actin filaments. Figure adapted with permission from ref. 7, Springer Nature Ltd.
Du and co-authors'approach involved the development of tunable AGE-crosslinked matrices and a robust quantitative method to characterize ECM immunity and cell signalling as a direct impact of mechanical forces and of crosstalk between the cells and the surrounding ECM. The authors began by showing that the crosslinking mediators LOX, AGEs and transglutaminase were highly expressed in human cirrhotic liver samples and in a mouse model of liver fibrosis. They then reconstructed AGE-crosslinked collagen matrices using a whole-liver-perfusion decellularization method that recapitulated the in vivo characteristics of the ECM in livers with late-stage fibrosis caused by AGE crosslinking. Subsequently, they used LC–MS/MS to track the degree of enzymatic (LOX and transglutaminase) and non-enzymatic (AGE) collagen crosslinking during fibrosis progression in the human cirrhotic liver samples and in mice. To examine the impact of AGE crosslinking on the biomechanical properties of the ECM, the authors employed glucose as a means to confer the model with a degree of AGE crosslinking comparable to that of liver ECM in late fibrosis, and used optical tweezers to map aberrant changes in the viscoelasticity, stiffness and stress-relaxation rate in the collagen matrix. The authors also investigated the mechanosensing of macrophages, which play a crucial role in the regulation of liver fibrosis3,8,9. They found that when macrophages were grown on an AGE-crosslinked matrix, the cells were unable to form protrusion structures and attach to neighbouring collagen fibrils. The resistance of AGE-crosslinked collagen fibrils to macrophage-mediated remodelling resulted in the inhibition of protrusion formation and in paxillin-adhesion formation in macrophages, as well as in the suppression of the cytoskeleton–histone-deacetylase-3 signalling pathway (Fig. 1). By using optical tweezers, the authors showed that the AGE-crosslinked fibrils resisted naturally occurring macrophage-mediated ECM remodelling. Cultured on this aberrant ECM, the macrophages displayed a reduced ability to recruit collagen fibrils, and such conditions triggered a type II immune response typical of liver cirrhosis. Moreover, the authors tested the therapeutic potential of the natural polyphenol antioxidant rosmarinic acid, which is known to inhibit the glycation of proteins and DNA10. Rosmarinic acid substantially reduced the crosslinking of collagen fibrils in the AGE-crosslinked matrices in vitro and in vivo, decreasing type II immune responses and alleviating liver fibrosis in mice with late-stage liver fibrosis.
Du and co-authors have previously reported that liver sinusoidal endothelial cells can present scar-degrading activity via the secretion of matrix metalloprotease-911. They developed an ECM degradation screening system that allowed for high-throughput analysis of collagen degradation in different cell populations seeded on collagen. Moreover, they showed that cells alleviated advanced liver fibrosis on splenic transplantation in mice11. Building on this progress, the authors now show that their collagen-based system can be used to investigate how cell–ECM crosstalk directly affects cell physiology7. The method can be further developed to investigate additional parameters associated with fibrosis progression in different tissues and health conditions. First, it can be applied to study the molecular response of other immune cell types to crosslinked ECM matrices, which is relevant to fibrosis in different tissues. It can also be used to explore the effect of collagen scaffolds on the angiogenic profile and immunological properties of dendritic cells in cancer and atherosclerosis12. Mucosal-associated invariant T cells, which play a role in liver fibrosis progression, can directly promote macrophage phenotypes with a pro-fibrogenic profile13, with LOX inhibiting the process. Thus, the method may support the characterization of different immune-cell–ECM crosstalk, including the extent of matrix degradation, cytoskeletal polarization and cellular signalling, and the molecular signatures9 relevant to different disease scenarios. Second, the method has implications for drug discovery. It can be used to assess the efficacy of approved drugs or compounds under development for the inhibition of AGE-mediated collagen crosslinking and for attenuation of fibrosis. Furthermore, it can be used to study fibrotic ECM in tumour microenvironments14 and autoimmune conditions, as well as to explore crosslinking by other agents, such as the enzyme lysyl oxidase like-2 (LOXL2)9. Third, the collagen matrix could serve as a biomimetic scaffold for tissue engineering. It would enable the study of the relative contribution of different cell types to tissue regeneration, as it can accommodate primary cells, tumour cells, stromal cells, stem cell-derived cells, endothelial cells, spheroids and organoids. Lastly, the method’s analytical tools may be adjusted to investigate the mechanical and chemical characteristics of collagen crosslinking by other agents15, and to assess their viscoelastic properties, specific bioactivity and biocompatibility. This would provide insights into the effects of cultured cells in different pathological states on function and mechanotransduction. Furthermore, the system can be optimized to integrate decellularized tissues and three-dimensional bioprinted scaffolds, and it may thus constitute a versatile system for investigating cell–ECM crosstalk using protocols for co-culture and organoid growth. However, the method's lack of sensitivity in capturing finely resolved spatial crosslinking cues within the ECM architecture hinders a comprehensive understanding of crosslinking patterns across diverse ECM microenvironments. Additionally, employing non-enzymatic crosslinking reactions to engineer precisely calibrated matrices raises concerns of an inherent lack of specificity, potentially leading to non-native crosslinks that could disrupt the ECM's structural and functional integrity, impacting the crucial interplay between the ECM and cellular components. Consequently, the envisioned matrices, although tunable, might not faithfully replicate the tissue-specific crosslinking that is critical for deciphering cell–ECM crosstalk. Despite the unclear role of enzymatic crosslinking in fibrosis and the lack of efficacy in clinical trials targeting LOX, the potential for effectively targeting abnormal crosslinking enzyme activity in fibrotic diseases should not be underestimated. The failure of clinical trials may be attributed to many factors (such as insufficient drug efficacy or selectivity, and inadequate targeted delivery) and the need for combination therapies. Notably, Du and colleagues' results show that AGE crosslinking of collagen in liver cirrhosis leads to dysfunctional mechanotransduction between the ECM and macrophages. Furthermore, that LOXL2-based therapy triggers the recruitment of matrix-metalloprotease-14-expressing macrophages to fibrotic sites9 suggests the possibility of combining ECM-crosslinking therapy with macrophage mobilization. Overall, Du and colleagues' method enables the precise characterization of the contributions of enzymatic and non-enzymatic ECM crosslinking, and may aid in the design of strategies for the treatment of fibrotic conditions across diseases.
DOI:https://doi.org/10.1038/s41551-023-01119-w
References:
[1] Cheemerla, S. & Balakrishnan, M. Clin. Liver Dis. 17, 365–370 (2021).
[2] Afratis, N. A. et al. Matrix Biol. 68-69, 167–179 (2018).
[3] Ortiz, C. et al. Curr. Tissue Microenviron. Rep. 2, 41–52 (2021).
[4] Luevano-Contreras, C. & Chapman-Novakofski, K. Nutrients 2, 1247–1265 (2010).
[5] Bansode, S. et al. Sci. Rep. 10, 3397 (2020).
[6] Fernando, D. H. et al. Int. J. Mol. Sci. 20, 5037 (2019).
[7] Lyu, C. et al. Nat. Biomed. Eng. https://doi.org/10.1038/s41551-023-01019-z (2023).
[8] Ramachandran, P. et al. Nature 575, 512–518 (2019).
[9] Klepfish, M. et al. Front. Immunol. 11, 480 (2020).
[10] Alrubaye, A., Motovali-Bashi, M. & Miroliaei, M. Sci. Rep. 11, 20605 (2021).
[11] Zhao, P. et al. Adv. Sci. 10, e2203315 (2023).
[12] Sprague, L. et al. Exp. Cell Res. 323, 7–27 (2014).
[13] Mabire, M. et al. Nat. Commun. 14, 1830 (2023).
[14] Mohan, V., Das, A. & Sagi, I. Semin. Cancer Biol. 62, 192–200 (2020).
[15] Adamiak, K. & Sionkowska, A. Int. J. Biol. Macromol. 161, 550–560 (2020).

